CPA Australia Survey: AI adoption by Mainland Chinese businesses escalates
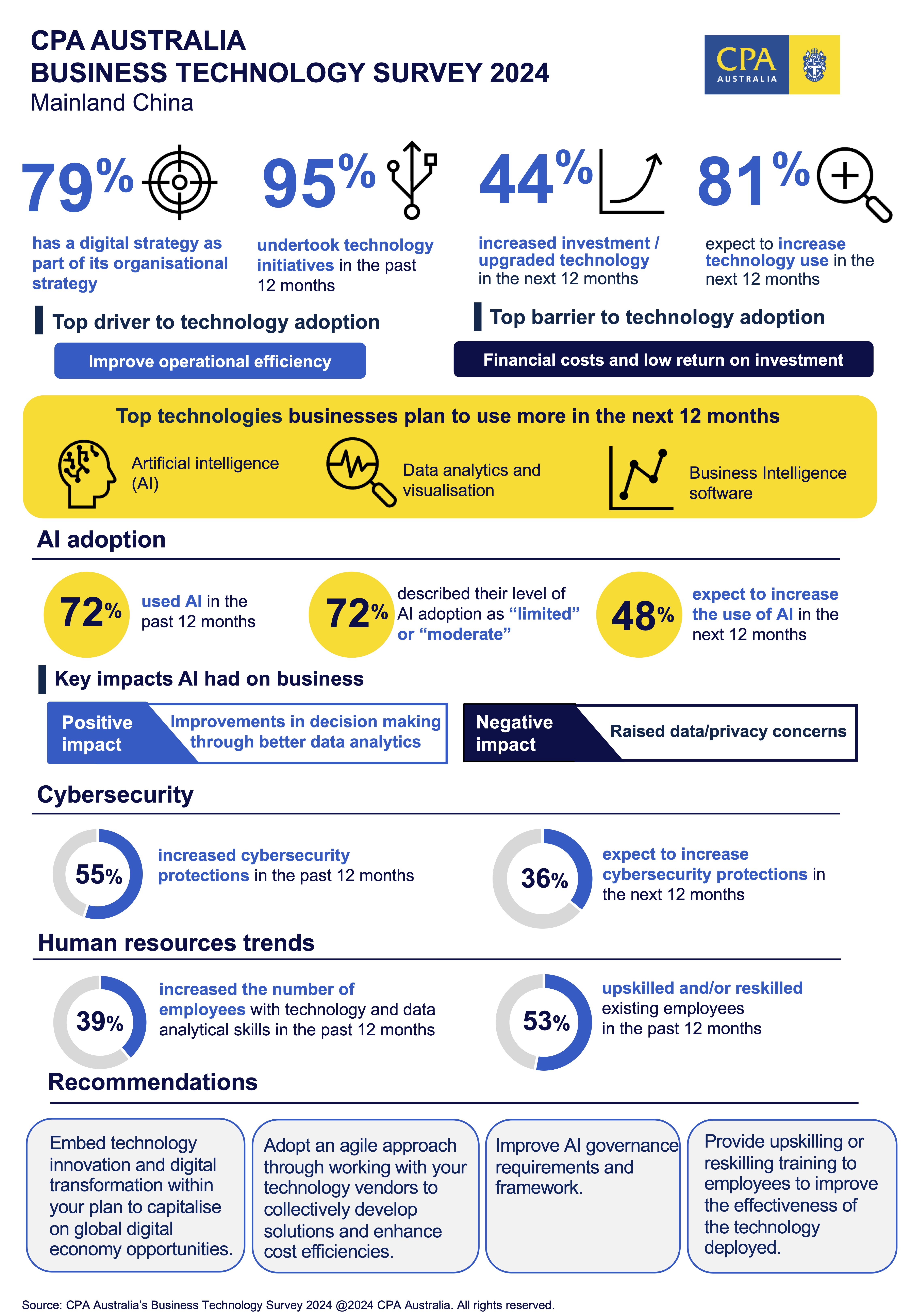
SHANGHAI, CHINA - Media OutReach Newswire - 22 August 2024 - The adoption of Artificial intelligence (AI) among Mainland Chinese companies has increased steadily over the past three years and is expected to surge in the next 12 months.

